


We adhere to first-class production and management, and constantly manufacture classics and perfection.

Biochemical extraction workshop: It covers an area of 1,400 square meters and can handle 15 tons of biochemical raw materials annually. The workshop is equipped with two Class C clean areas with independent clean air-conditioning systems, which are respectively used for production before and after sterilization and filtration. It is equipped with raw material cold storage, semi-finished product cold storage, intermediate product cold storage and extract cold storage. With colloid mills, heating tanks, ultrafilters and ultrafiltration systems and other equipment, the workshop is highly automated.

Hydro-acupuncture workshop: It has an aseptic filling linkage production line with an annual output of 30 million small-volume injections. It uses advanced equipment at home and abroad, such as a washing-drying-irrigation linkage line, a preparation filter system equipped with a weighing module, a water bath sterilizer and pure steam sterilization At the same time, automatic foreign body inspection machines imported from Japan have been introduced. The workshop has a Class C clean area and a Class B clean area (the filling area is B+A). The design of the workshop is reasonable, the performance of the equipment is stable, and it meets the requirements of GMP.

The warehouse is equipped with a cool warehouse, a normal temperature warehouse and a hazardous goods warehouse. And the warehouse is equipped with clean sampling rooms, various protective facilities and temperature and humidity monitoring devices. The bottom traying pattern is adopted for the storage of materials, in which they are stored according to storage conditions and validity period to ensure absolute safety.

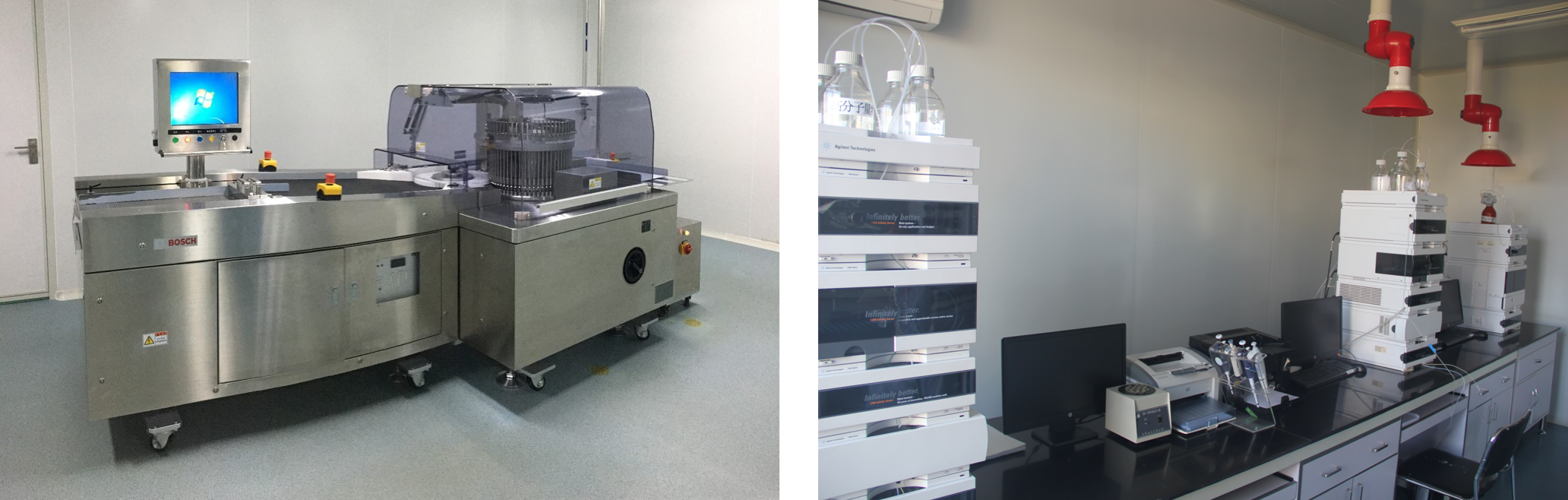
We have selected advanced equipment and instruments at home and abroad for the production of key equipment and main instruments for inspection, and has formulated equipment use and cleaning operation procedures as well as preventive maintenance plans and operation procedures. The design, selection and maintenance of the equipment meet the requirements of product production, and the inspection and maintenance for it are carried out on a regular basis.

We have established a drug production quality management system, covering facilities and equipment, production management, quality management, material control, verification management, document management, storage, shipment and other links. there are quality assurance rooms and quality control laboratories, with each department having clear responsibilities. Risk management procedures have been made and risk management activities are also carried out in accordance with relevant requirements; All this ensures that the whole process of drug production continuously meets the legal requirements.
We have established a pharmacovigilance system document with an adverse reaction monitoring room, which is an independent department and parallel to the Quality Department, the Production Department and other departments. It has a clear organizational structure, where each department has its clear responsibilities; and a drug safety problem handling mechanism together with a drug safety committee with the participation of multiple departments has also been established. The Periodic Safety Update Report (PSUR) for Spleen Polypeptide Injection was completed in 2013 and 2018 respectively and assessed well by the Provincial Adverse Reaction Center.
We have carried out ADR monitoring of post-marketing drugs, during which it actively collects, tracks and analyzes ADR information, and takes timely risk control measures.





